At GreyRigge, we routinely use a Quality by Design (QbD) approach where we design experiments and analyse data through statistical analyses. This drives improved understanding, safety, efficacy, toxicology of products as well as assay and process control through building design space knowledge. Employing these platforms already allows us to reliably predict outcomes from well-designed data driven experiments. We use this to help clients efficiently and compliantly move their medicines from development through to commercialisation, often, with accelerated results.
A goal for deploying AI in medicines development and its manufacturing is to move away from a "quality by testing" paradigm to a "quality by design" and "quality by prediction" model.
To illustrate, we have designed the GreyRigge AI Ecosystem:
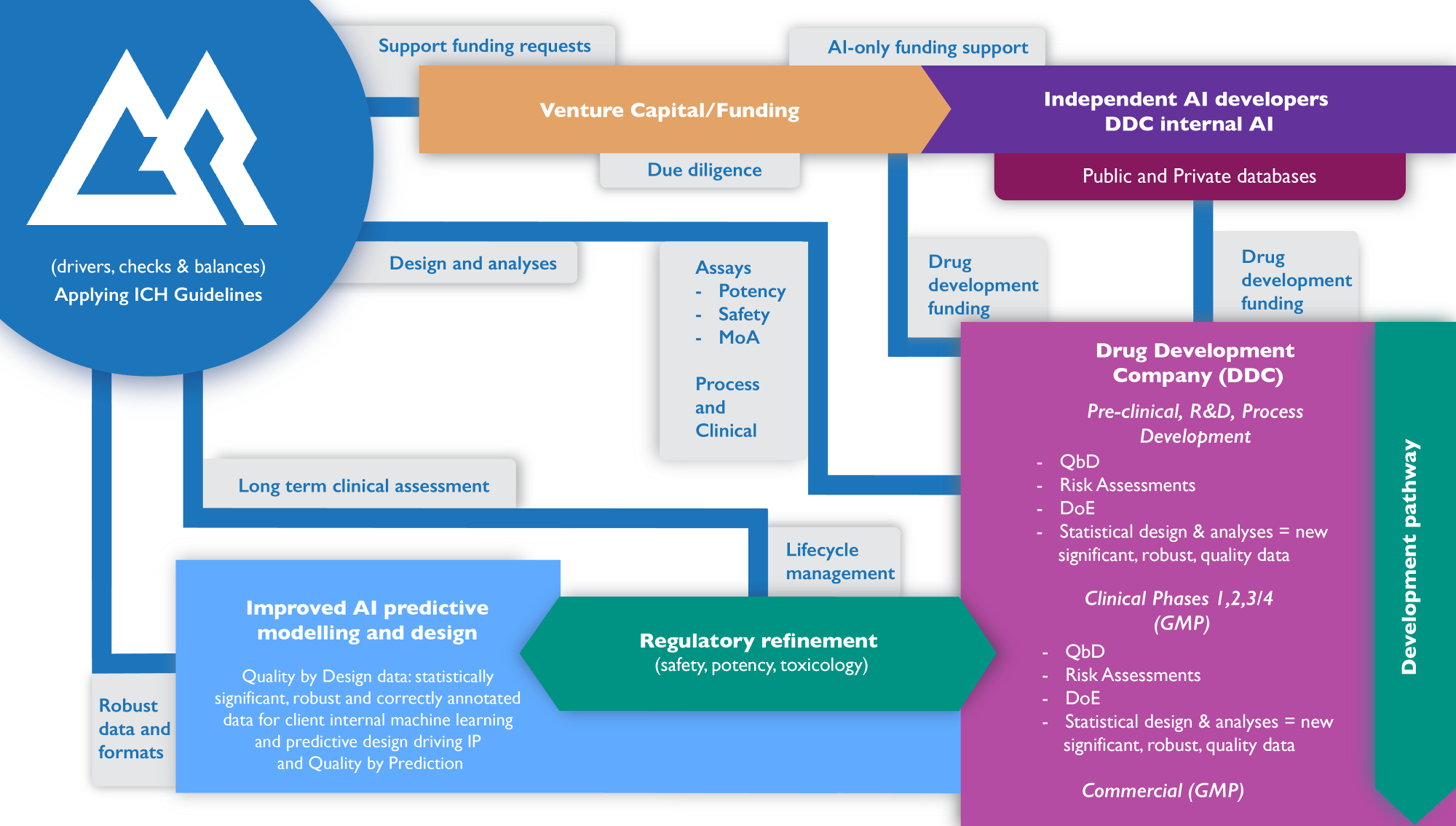
Leading AI Through QbD
Quality by Design (QbD) to Quality by Prediction: wherever you are in your product development pathway, QbD comes first in generating powerful significantly relevant data for your product development and/or to improve your machine learning.

Deployed correctly, applying AI in biotech should be an accelerator. But rigorous assessment of data from and across process, assay, preclinical safety, and through to the clinical designs and its associated clinical outcomes is required. AI accuracy relies on the quality of data it can use. This is because it is a component of the coding for the machine learning process. High quality statistically designed approaches and analysed data need to feed back into the machine learning for individual companies to improve predictive modelling. A number of CDMOs have already put this approach in place for accelerating assay and process assessment during development.
We embrace the use of AI in Biotech and can point to numerous development cycles where we have been involved. It remains that every step of the development process is meticulously tested, documented and proven through experimentation with good statistical design and analyses. This is where we add value. We are experts on design approaches and navigating data that can be used for coding that will help AI engineers develop algorithms to predict results and subsequently put in place the mechanics of testing. Documentation and process does not disappear. In fact, the level of resilience and accuracy continue at the highest level to ensure that erroneous results and outliers are identified and addressed.
If the rigorous testing with quality based statistical approaches are used per ICH guidelines, this will accelerate design to clinical outcome and support regulatory filing, but with reduced testing. Once in place, AI will become a true accelerator of safe and efficacious product development and drive Quality by Prediction.
Whether you use AI or not, GreyRigge support spans the complete therapeutic development continuum using a Quality by Design approach. This ensures that the quality of data sets and their analyses are optimal.
Investors
(VCs, PE etc) needing to complete due diligence and assess AI and QbD requirements in prospective investments.
AI platform developers
Wanting to understand the application and role of QbD and use of DoE in order to aide machine learning so that robustand applicable AI tools can be built for Medicinal Product Development Companies.
Drug Development Companies
Across the entire process, including Assays, Quality Assurance/Control, Regulatory and Clinical through to commercialisation. We maximise the robustness of data; format feedback for AI across dataset machine learning; improve prognostics and diagnostics to help better define clinical design parameters; build testeddata driven submissions for regulatory applications, along with long-term follow-up data to drive confidence and reduce workload for subsequent submissions; and aid lifecycle management. Our deep skills and experience reduce risks and accelerate timelines of development and commercialisation.
